Sridhar Rao Laboratory
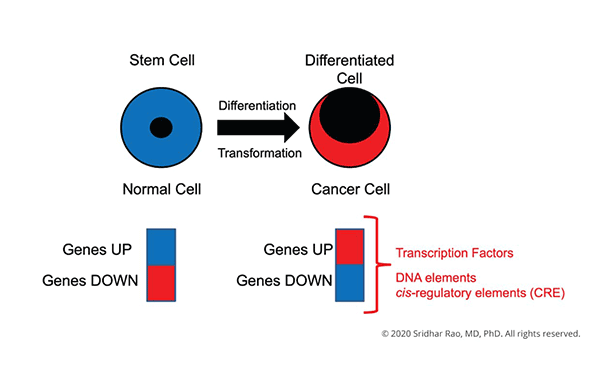
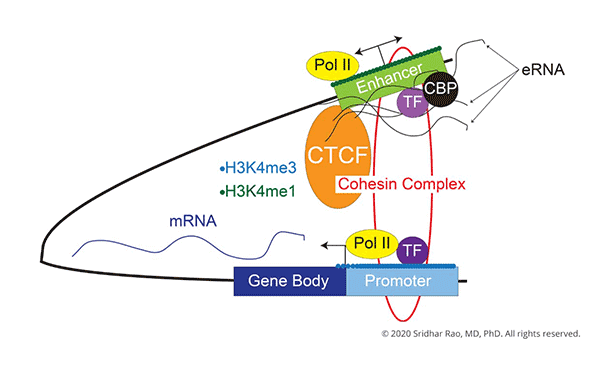
The central focus of my laboratory is to understand how changes in gene expression ultimately lead to cell-fate decisions.
Resources
View selected publications and datasets in the resources section.
Full Publication List
Complete List of Published Work in NCBI My Bibliography.