TARGETING THE METABOLIC REGULATOR SIRT5 IN ACUTE MYELOID LEUKEMIA. AML is an aggressive hematologic malignancy with <30% long-term survival. The current therapy standard, chemotherapy alone or combined with allogeneic stem cell transplant, has not changed for decades. Despite initial responses, most patients eventually relapse, suggesting persistence of leukemia initiating cells in protective niches. Inhibitors of FLT3 or mutant isocitrate dehydrogenase 1/2 (IDH1/2) have expanded therapy options and validated the paradigm of genotypedirected therapy. However, even with these new drugs, relapse is common and frequently due to selection of subclones with resistance mutations in the drug target. Unlike FLT3 and IDH1/2 inhibitors, the BCL2 inhibitor venetoclax is active in multiple AML genotypes, indicating that targeting shared vulnerabilities in a genotype-agnostic manner can be effective. Unfortunately, many venetoclax-induced responses are not durable as leukemia cells adapt by activating alternative anti-apoptosis mechanisms or by reprogramming mitochondrial metabolism.
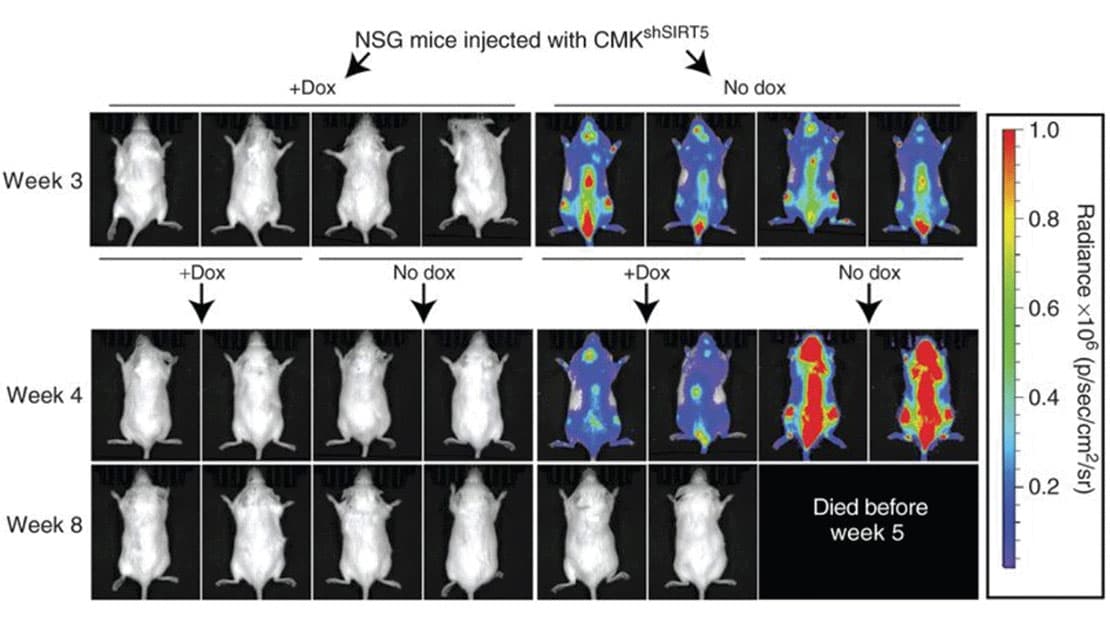 SIRT5 KD attenuates leukemia in a xenograft model.
SIRT5 KD attenuates leukemia in a xenograft model.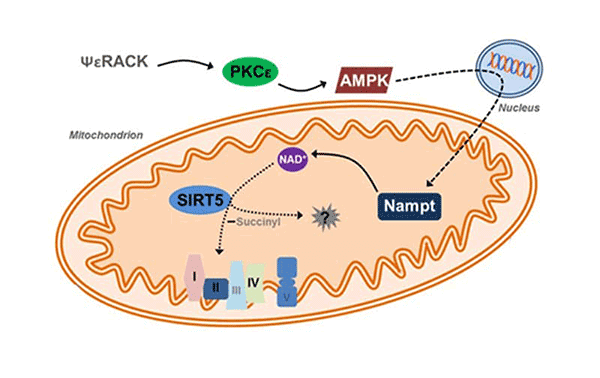 SIRT5 as a mitochondria-localized enzyme belonging to a family of NAD+ dependent histone
SIRT5 as a mitochondria-localized enzyme belonging to a family of NAD+ dependent histone 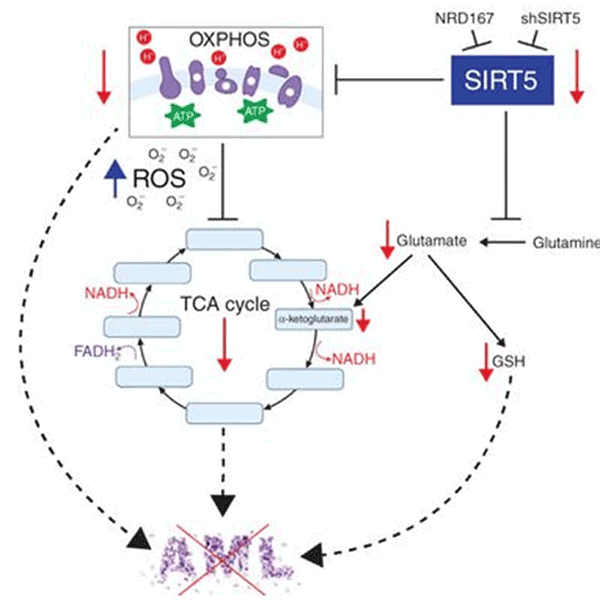 Schematic depicting metabolic consequences of SIRT5 KD.
Schematic depicting metabolic consequences of SIRT5 KD.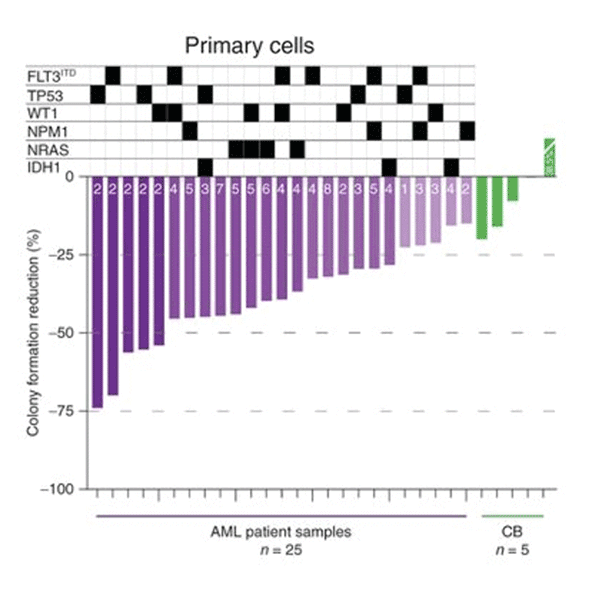 AML cells selectively dependent on SIRT5.
AML cells selectively dependent on SIRT5.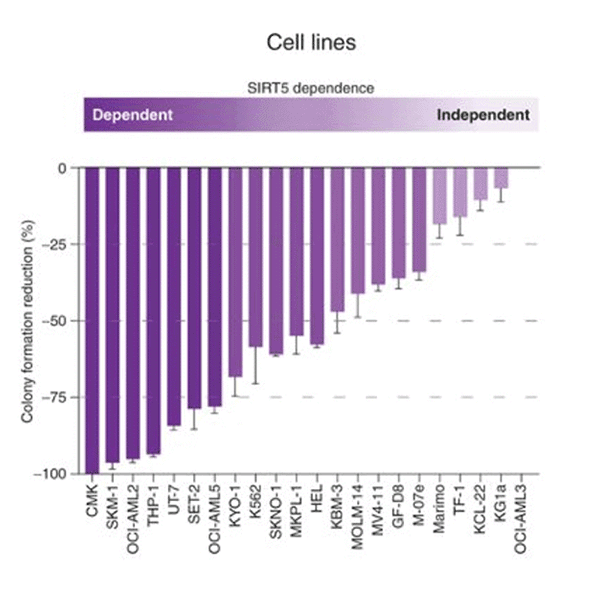 AML cells are slectively dependent on SIRT5
AML cells are slectively dependent on SIRT5Microenvironmental protection, intra-tumoral heterogeneity and metabolic flexibility limit the utility of current AML therapies. To identify new therapy targets in AML, we adapted an shRNA screen for testing primary AML cells under bone marrow microenvironment-like conditions. We discovered that many AML patient samples are highly dependent on SIRT5, while normal CD34+ cells are not. SIRT5 is a lysine deacylase implicated in the regulation of energy metabolic pathways, including oxidative phosphorylation (OXPHOS), fatty acid ?-oxidation and glycolysis. SIRT5 knockdown (KD) reduces growth and increases apoptosis in most AML cell lines, with consistent results upon disruption of SIRT5 using CRISPR/Cas9 or NRD167, a novel cell-permeable SIRT5 inhibitor. Genetic absence of Sirt5 impairs in vitro transformation of mouse hematopoietic cells by several myeloid oncogenes, including MLL-AF9, and attenuates leukemogenesis in vivo. At a biochemical level, SIRT5 KD or inhibition with NRD167 is associated with reduced OXPHOS, reduced glutathione levels and increased mitochondrial superoxide, suggesting that AML cells depend on SIRT5 to maintain redox homeostasis. Sirt5-/- mice are viable with minor metabolic abnormalities, suggesting that in vivo inhibition of SIRT5 would be tolerated.
We hypothesize that SIRT5 is a therapy target in AML and will test this in three Specific Aims: (1) Identify and validate SIRT5-regulated metabolic pathways in normal and AML stem and progenitor cells. (2) Identify biomarkers of sensitivity to SIRT5 inhibition in primary AML cells. (3) Identify a potent, selective, and bioavailable SIRT5 inhibitor, starting from the NRD167 tool compound. Our work will rigorously test whether SIRT5 is a therapy target in AML, clarify the mechanisms underlying SIRT5 dependence, and identify potent and selective SIRT5 inhibitors for future clinical development.