Robert Montgomery, MD
Over the past year we have 1) demonstrated that FVIII is made in endothelial cells; 2) completed enrollment of our historical type 1 Von Willebrand disease cohort of 500 families done across the USA; 3) initiated a prospective study of newly diagnosed VWD patients across the USA; 4) did BM TxP with gene therapy of the stem cells to direct FVIII production in platelets; and 5) initialed studies to study acquired abnormalities of von Willebrand factor in newborns having open heart surgery for complex congenital heart disease.
While scientists have recognized for years that liver transplant can cure hemophilia and concluded that FVIII must be made in the liver hepatocyte. Using various mouse models that we developed, studies were possible to block the FVIII production in a single type of cell. For example we can remove the FVIII functional gene from all hepatocytes. Interestingly, the FVIII level in the mouse plasma was normal and not affected – thereby proving that hepatocytes didn’t make FVIII. We then did further “tissue-specific” knockout or elimination of the functional FVIII gene from other cells and thereby proved that if you stopped FVIII production in endothelial cells, there was no FVIII in plasma and thereby proved that FVIII was produced in endothelial cells where it was stored and released physiologically with its companion molecule von Willebrand factor.
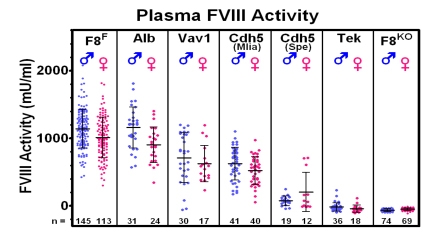
This figure demonstrates that crossing our mouse with a “floxed” FVIII gene with mice with tissue-specific “Cre-recombinase” demonstrate no reduction of FVIII in the albumin-Cre (hepatocyte-specific) mouse, but total elimination of plasma FVIII in the Tek-Cre (endothelial cell specific) mouse.
We have finished the 7th year of a multinational program project that is focused on von Willebrand Disease (VWD). VWD is probably the most common hereditary bleeding disorder and may affect 1 in 50 to 1 in 1000 of our general population. In studying our cohort of 500 families with the common form of VWD, type 1 VWD, we found that not all of those subjects continue to have abnormal levels of VWF. We do find that most have a gene mutation in their VWF gene that can be associated with low VWF in other family members. We have recently found a severe form of VWD in which their blood VWF doesn’t bind to exposed collagen after blood vessels are damaged. Current testing as done widely in the United States misses this form of VWD, yet these patients would benefit from VWF replacement therapy. Also we have shown that certain VWF laboratory test (ristocetin-cofactor) is not accurate in as many as 67% of African Americans – they might be assumed to have VWD when they really are normal.
Since our historic diagnosis of VWD might have diagnostic problems, might be develop algorithms that correctly diagnose VWD patients more correctly and provide individualized advice on need for treatment when bleeding is encountered.
For hemophilia we have devised a strategy of gene therapy where we would take a hemophilia patients bone marrow and do gene therapy on his hematopoietic stem cells (blood stem cells) so that the FVIII (or FIX) gene is turned on only in platelets where it is stored in storage granules. If blood vessels are injured the platelet sticks to the site because of VWF and the platelets activate and release the FVIII that was put there by gene therapy and the FVIII accelerates clotting and stops the clinical bleeding. To see if there is any thrombosis risk, we have done similar bone marrow transplants with platelet FVIII into mice with a thrombotic propensity. Adding the platelet FVIII does NOT seem to increase the likelihood of clinical thrombosis. This suggests this approach may be safe.
Our lab has shown that heart valve problems result in the loss of the most active “large” forms of VWF that are most likely to stop clinical bleeding. When infants in their first month of life have major heart surgery for complex congenital heart disease, they sometimes encounter excess abnormal bleeding. Our studies are directed at defining if this bleeding is caused by abnormal VWF and if so, could VWF replacement be optimized to control hemorrhage.
These 5 studies are directed at increasing our understanding of both bleeding and clotting disorders and their appropriate clinical management.
Selected Publications
Schroeder, J.A., Kuether, E.A., Fang, J., Jing, W., Weiler, H., Wilcox, D.A., Montgomery, R.R., Shi, Q. Thromboelastometry assessment of hemostatic properties in various murine models with coagulopathy and the effect of factor VIII therapeutics. J. Thromb. Haemost. 2021 Jul 10. PMID: 34245090.
Digiandomenico S, Conley SF, Johnson VP, Christopherson PA, Haberichter SL, Zhang J, Simpson P, Abshire TC, Montgomery RR, Flood VH. Screening for von Willebrand disease does not impact posttonsillectomy bleeding in a low-risk population. Pediatr Blood Cancer. 2021 Dec;68(12):e29371. doi: 10.1002/pbc.29371. Epub 2021 Oct 4. PMID: 34606172; PMCID: PMC8919995.
Kalot MA, Husainat N, El Alayli A, Abughanimeh O, Diab O, Tayiem S, Madoukh B, Dimassi AB, Qureini A, Ameer B, Eikenboom JCJ, Giraud N, McLintock C, McRae S, Montgomery RR, O'Donnell JS, Scappe N, Sidonio RF, Brignardello-Petersen R, Flood VH, Connell NT, James PD, Mustafa RA. von Willebrand factor levels in the diagnosis of von Willebrand disease: a systematic review and meta-analysis. Blood Adv. 2022 Jan 11;6(1):62-71. doi: 10.1182/bloodadvances.2021005430. PMID: 34610118; PMCID: PMC8753202.
Sadler B, Minard CG, Haller G, Gurnett CA, O'Brien SH, Wheeler A, Jain S, Sharma M, Zia A, Kulkarni R, Mullins E, Ragni MV, Sidonio R, Dietrich JE, Kouides PA, Di Paola J, Srivaths L. Whole-exome analysis of adolescents with low VWF and heavy menstrual bleeding identifies novel genetic associations. Blood Advances 2022 Jan 25;6(2):420-428. PMID: 34807970; (PMCID: PMC8791588).
Shi Q, Fahs SA, Mattson JG, Yu H, Perry CL, Morateck PA, Schroeder JA, Rapten J, Weiler H, Montgomery RR. A novel mouse model of type 2N VWD was developed by CRISPR/Cas9 gene editing and recapitulates human type 2N VWD. Blood Adv. 2022 May 10;6(9):2778-2790. doi: 10.1182/bloodadvances.2021006353. PMID: 35015821; PMCID: PMC9092403.
Kanaji S, Morodomi Y, Weiler H, Zarpellon A, Montgomery RR, Ruggeri ZM, Kanaji T. The impact of aberrant von Willebrand factor-GPIbα interaction on megakaryopoiesis and platelets in humanized type 2B von Willebrand disease model mouse. Haematologica. 2022 Sep 1;107(9):2133-2143. doi: 10.3324/haematol.2021.280561. PMID: 35142156; PMCID: PMC9425322.
Christopherson PA, Haberichter SL, Flood VH, Perry CL, Sadler BE, Bellissimo DB, Di Paola J, Montgomery RR; Zimmerman Program Investigators. Molecular pathogenesis and heterogeneity in type 3 VWD families in U.S. Zimmerman program. J Thromb Haemost. 2022 Jul;20(7):1576-1588. doi: 10.1111/jth.15713. Epub 2022 Apr 6. PMID: 35343054.
Lavin M, Christopherson P, Grabell J, Abshire T, Flood V, Haberichter SL, Lillicrap D, O'Donnell JS, Montgomery RR, James PD. Longitudinal bleeding assessment in von Willebrand disease utilizing an interim bleeding score. J Thromb Haemost. 2022 Oct;20(10):2246-2254. doi: 10.1111/jth.15807. Epub 2022 Jul 26. PMID: 35780487; PMCID: PMC10193460.
Christopherson PA, Haberichter SL, Flood VH, Sicking UO, Abshire TC, Montgomery RR; Zimmerman Program Investigators. Ristocetin dependent cofactor activity in von Willebrand disease diagnosis: Limitations of relying on a single measure. Res Pract Thromb Haemost. 2022 Oct 5;6(7):e12807. doi: 10.1002/rth2.12807. PMID: 36381287; PMCID: PMC9637542.
Doherty D, Grabell J, Christopherson PA, Montgomery RR, Coller BS, Lavin M, O'Donnell JS, James PD; Zimmerman Program Investigators. Variability in International Society on Thrombosis and Haemostasis-Scientific and Standardization Committee endorsed Bleeding Assessment Tool (ISTH-BAT) score with normal aging in healthy females: contributory factors and clinical significance. J Thromb Haemost. 2023 Apr;21(4):880-886. doi: 10.1016/j.jtha.2022.11.045. Epub 2022 Dec 27. PMID: 36696194.
